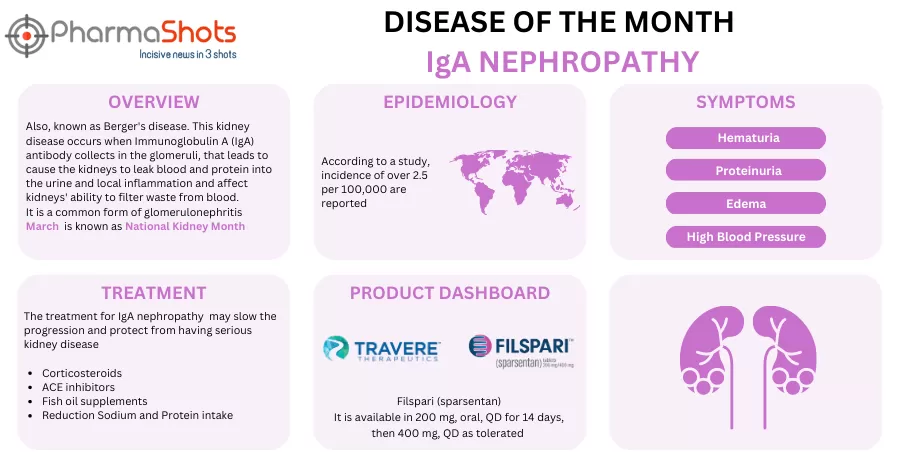
Travere Therapeutics’ Filspari (sparsentan) Receives the US FDA’s Full Approval for Treating IgA Nephropathy
Shots:
- The US FDA has granted full approval to Filspari (400mg) for treating IgAN, based on the P-III (PROTECT) trial assessing the drug vs irbesartan (300mg) in subjects (n=404) aged ≥18yrs. It received accelerated approval in Feb 2023
- Final 2yrs. ITT analysis showed reduction in the rate of kidney function decline at wk.110. The mean eGFR slope was -3.0 mL/min/1.73 m2/year vs -4.2 mL/min/1.73 m2/year, with a significant treatment effect of 1.2 mL/min/1.73 m²/year; additional benefits showed improved eGFR over time, with a 3.8 mL/min/1.73 m² greater mean change
- Safety was well-tolerated and consistent in all the studies. An sNDA to the US FDA is planned for a potential modification to the liver-monitoring REMS
Ref: Travere Therapeutics | Image: Travere Therapeutics
Related News:- CSL Vifor and Travere Therapeutics’ FILSPARI (sparsentan) Received European Commission Approval Against IgA Nephropathy
PharmaShots! Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com
Click here to read the full press release
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.